<%
Dim currentFolder, name, email
currentFolder = "Laboratory of Developmental Biology Protein Glycobiology Section"
if currentFolder = "chd Staff" then
'response.write(currentFolder)
%>
<%
' Function IsEmailValid(strEmail)
' Action: checks if an email is correct.
' Parameter: strEmail - the Email address
' Returned value: on success it returns True, else False.
Function IsEmailValid(strEmail)
Dim strArray
Dim strItem
Dim i
Dim c
Dim blnIsItValid
' assume the email address is correct
blnIsItValid = True
' split the email address in two parts: name@domain.ext
strArray = Split(strEmail, "@")
' if there are more or less than two parts
If UBound(strArray) <> 1 Then
blnIsItValid = False
IsEmailValid = blnIsItValid
Exit Function
End If
' check each part
For Each strItem In strArray
' no part can be void
If Len(strItem) <= 0 Then
blnIsItValid = False
IsEmailValid = blnIsItValid
Exit Function
End If
' check each character of the part
' only following "abcdefghijklmnopqrstuvwxyz_-."
' characters and the ten digits are allowed
For i = 1 To Len(strItem)
c = LCase(Mid(strItem, i, 1))
' if there is an illegal character in the part
If InStr("abcdefghijklmnopqrstuvwxyz_-.", c) <= 0 And Not IsNumeric(c) Then
blnIsItValid = False
IsEmailValid = blnIsItValid
Exit Function
End If
Next
' the first and the last character in the part cannot be . (dot)
If Left(strItem, 1) = "." Or Right(strItem, 1) = "." Then
blnIsItValid = False
IsEmailValid = blnIsItValid
Exit Function
End If
Next
' the second part (domain.ext) must contain a . (dot)
If InStr(strArray(1), ".") <= 0 Then
blnIsItValid = False
IsEmailValid = blnIsItValid
Exit Function
End If
' check the length oh the extension
i = Len(strArray(1)) - InStrRev(strArray(1), ".")
' the length of the extension can be only 2, 3, or 4
' to cover the new "info" extension
If i <> 2 And i <> 3 And i <> 4 Then
blnIsItValid = False
IsEmailValid = blnIsItValid
Exit Function
End If
' after . (dot) cannot follow a . (dot)
If InStr(strEmail, "..") > 0 Then
blnIsItValid = False
IsEmailValid = blnIsItValid
Exit Function
End If
' finally it's OK
IsEmailValid = blnIsItValid
End Function
%>
<% end if %>
<% if currentFolder = "emailForm" then%>
<%
function getEmail(name)
end function
%>
[an error occurred while processing this directive]
<%
name = Request.QueryString("name")
email = getEmail(name)
if Not IsEmailValid(email) then
response.write("Send E-Mail to: ")
response.write(name + " Valid e-mail address not found.
")
else
response.write("Send E-Mail to: ")
response.write(name + "
")
''response.write(email + "
")
validSendEmailFound = true
end if
%>
Curriculum Vitae
PROTEIN
GLYCOBIOLOGY SECTION
|
DR. KENNETH
KRAMER
Section
Head
Bldg. 10, Room 8N228
Bethesda, Maryland 20892
Email: kramerk2@nhlbi.nih.gov
|
EDUCATION
|
1998-2004
|
Postdoctoral
Fellow, Huntsman Cancer Institute, University of Utah; Salt Lake City, UT
(Mentor: H. Joseph Yost)
|
|
1995-1996
|
Adjunct
Instructor, Department of Biology, Raymond Walters College, University of
Cincinnati; Cincinnati, OH (Chairman: Donald
M. Meismer)
|
|
1991-1997
|
PhD,
Department of Cell Biology, Neurobiology, and Anatomy, University of
Cincinnati; Cincinnati, OH (Mentor: Richard L. Drake)
|
|
1987-1991
|
BS,
Biology, University of Dayton; Dayton, OH
|
EXTERNAL PROFESSIONAL ACTIVITIES
|
2004-2006
|
Chair, Advisory
Committee for Young Anatomists, American Association of Anatomists
|
|
2004-present
|
Scientific
Consultant, Discovery Genomics; Minneapolis, MN
|
|
2004-2006
|
Member, Membership
Committee, American Association of Anatomists
|
|
2004
|
Symposium Chair,
“Sweet talk: Heparan sulfate proteoglycans in developmental cell signaling.”
Experimental Biology. Washington, DC.
|
SELECTED RECENT PUBLICATIONS
|
Kramer, K.L., and H.J. Yost. (2003). Heparan sulfate core proteins in cell-cell
signaling. Annual
Review of Genetics 37, 461-84.
|
|
Kramer, K.L., and Yost, H. J. (2003). Cardiac
left-right development: Are the early steps conserved? In Cold Spring Harbor Symposia on Quantitative
Biology: The Cardiovascular System (Cold Spring Harbor, NY,
Cold Spring Harbor Laboratory), pp. 37-43.
|
|
Kramer, K.L., Barnette, J.E., and H.J. Yost. (2002). PKCg regulates syndecan-2 inside-out
signaling during Xenopus left-right
development. Cell 111, 981-90.
|
|
Kramer, K.L., and H.J. Yost. (2002). Ectodermal
syndecan-2 mediates left-right axis formation in migrating mesoderm as a
cell-nonautonomous Vg1 cofactor. Developmental
Cell 2, 115-24.
|
Research
Summary
My long-term research
interest is to understand the genes and mechanisms that regulate cardiac cell
induction and migration during early vertebrate embryogenesis. Vertebrate
cardiogenesis begins during gastrulation, an early stage of development in
which large groups of cells coordinately move to give rise to the ectodermal,
mesodermal and endodermal germ layers. By the end of gastrulation, mesodermal
cardiac progenitor cells have also received information that determines their
developmental fate. The gastrula-stage genes involved in the specification of
cell identity and the direction of cell migration are still being identified,
and little is known about the relationship between cell specification and
movement. Conclusive evidence does demonstrate that cell-cell signaling is
integral in controlling both cell motility and cell fate.
Recent
studies in a number of model systems have demonstrated that all three of the
cell-cell signaling pathways that regulate early cardiogenesis (TGFβ, Wnt, and FGF) are regulated by heparan sulfate. Heparan
sulfate is an unbranched sugar chain consisting of repeateddisaccharides
that are modified by sulfation and epimerization during synthesis in the golgi.
The result is a finely structured chain with specific protein binding
affinities. Heparan sulfate is covalently attached to core proteins in the
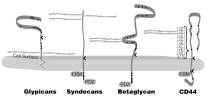
extracellular matrix and at the cell surface, and proteins to which heparan
sulfate attaches are referred to as heparan sulfate proteoglycans (HSPGs). At
the cell surface, the predominant HSPGs belong to two families of core
proteins: transmembrane syndecans and glycophosphatidylinositol (GPI)-linked
glypicans. Characterizing both the developmental and cell biological function
of HSPGs during early zebrafish development is the focus of my laboratory.
Because many steps in zebrafish embryogenesis are similar to those in humans,
the mechanisms and modifiers that I identify may be applicable to better
understanding a wide range of cell-cell signaling events in development and
disease.
Project 1: What is the role of HS core
proteins during gastrulation?
From Drosophila to mouse,
all three cell-cell signaling pathways that regulate early cardiogenesis are in
turn regulated by HSPGs. Consequently, I think the question is not if HSPGs
regulate cardiogenesis, but which specific core proteins are involved and how
do they function? The partial assembly of the zebrafish genome in the last year
has allowed me to clone apparently all 15 of the zebrafish HS cell surface core
proteins. Interestingly, at least 13 are expressed at the beginning of
gastrulation. Of these, only 2 have been described, and they both have distinct
gastrula stage defects in cell migration.
Project 2: Is the HS
fine structure a temporally and spatially permissive sugar code?
Covalently attached
to each core protein is an unbranched chain of 50-100 disaccharide repeats; and
each HS disaccharide can be modified at up to six positions, leading to an
extraordinary level of sequence diversity. Commonly referred to as its fine
structure, the pattern of HS modification over 2-6 disaccharides creates a
specific ligand binding site. In many cases, a cell can only respond to a
cell-cell signaling molecule if it has an appropriate HS fine structure at its
cell surface. Recent results have shown that the HS fine structure changes
during development, suggesting that a cell’s developmental fate is determined
in part by which cell-cell signaling molecules bind to its HS fine structure.
Project 3: Does the core
protein specify its attached HS fine structure?
The current model for
HS synthesis is that the HS fine structure is determined by the complement of
sulfotransferases expressed within the golgi of each cell, regardless of the
core protein to which it might attach. This model is challenged by observations
that modifications in the core protein can alter the interaction of HSPGs with
signaling pathways. However, the HS fine structure might be regulated by the
transition of the core protein through distinct combinations of
sulfotransferase isoforms within the golgi.
<% end if %>
<%
if currentFolder <> "emailForm" and currentFolder <> "LCE Staff" then
'response.write "dummy statement"
%>
Curriculum Vitae
PROTEIN
GLYCOBIOLOGY SECTION

|
DR. KENNETH
KRAMER
Section
Head
Bldg. 10, Room 8N228
Bethesda, Maryland 20892
Email: kramerk2@nhlbi.nih.gov
|
EDUCATION
|
1998-2004
|
Postdoctoral
Fellow, Huntsman Cancer Institute, University of Utah; Salt Lake City, UT
(Mentor: H. Joseph Yost)
|
|
1995-1996
|
Adjunct
Instructor, Department of Biology, Raymond Walters College, University of
Cincinnati; Cincinnati, OH (Chairman: Donald
M. Meismer)
|
|
1991-1997
|
PhD,
Department of Cell Biology, Neurobiology, and Anatomy, University of
Cincinnati; Cincinnati, OH (Mentor: Richard L. Drake)
|
|
1987-1991
|
BS,
Biology, University of Dayton; Dayton, OH
|
EXTERNAL PROFESSIONAL ACTIVITIES
|
2004-2006
|
Chair, Advisory
Committee for Young Anatomists, American Association of Anatomists
|
|
2004-present
|
Scientific
Consultant, Discovery Genomics; Minneapolis, MN
|
|
2004-2006
|
Member, Membership
Committee, American Association of Anatomists
|
|
2004
|
Symposium Chair,
“Sweet talk: Heparan sulfate proteoglycans in developmental cell signaling.”
Experimental Biology. Washington, DC.
|
SELECTED RECENT PUBLICATIONS
|
Kramer, K.L., and H.J. Yost. (2003). Heparan sulfate core proteins in cell-cell
signaling. Annual
Review of Genetics 37, 461-84.
|
|
Kramer, K.L., and Yost, H. J. (2003). Cardiac
left-right development: Are the early steps conserved? In Cold Spring Harbor Symposia on Quantitative
Biology: The Cardiovascular System (Cold Spring Harbor, NY,
Cold Spring Harbor Laboratory), pp. 37-43.
|
|
Kramer, K.L., Barnette, J.E., and H.J. Yost. (2002). PKCg regulates syndecan-2 inside-out
signaling during Xenopus left-right
development. Cell 111, 981-90.
|
|
Kramer, K.L., and H.J. Yost. (2002). Ectodermal
syndecan-2 mediates left-right axis formation in migrating mesoderm as a
cell-nonautonomous Vg1 cofactor. Developmental
Cell 2, 115-24.
|

Research
Summary
My long-term research
interest is to understand the genes and mechanisms that regulate cardiac cell
induction and migration during early vertebrate embryogenesis. Vertebrate
cardiogenesis begins during gastrulation, an early stage of development in
which large groups of cells coordinately move to give rise to the ectodermal,
mesodermal and endodermal germ layers. By the end of gastrulation, mesodermal
cardiac progenitor cells have also received information that determines their
developmental fate. The gastrula-stage genes involved in the specification of
cell identity and the direction of cell migration are still being identified,
and little is known about the relationship between cell specification and
movement. Conclusive evidence does demonstrate that cell-cell signaling is
integral in controlling both cell motility and cell fate.
Recent
studies in a number of model systems have demonstrated that all three of the
cell-cell signaling pathways that regulate early cardiogenesis (TGFβ, Wnt, and FGF) are regulated by heparan sulfate. Heparan
sulfate is an unbranched sugar chain consisting of repeateddisaccharides
that are modified by sulfation and epimerization during synthesis in the golgi.
The result is a finely structured chain with specific protein binding
affinities. Heparan sulfate is covalently attached to core proteins in the
extracellular matrix and at the cell surface, and proteins to which heparan
sulfate attaches are referred to as heparan sulfate proteoglycans (HSPGs). At
the cell surface, the predominant HSPGs belong to two families of core
proteins: transmembrane syndecans and glycophosphatidylinositol (GPI)-linked
glypicans. Characterizing both the developmental and cell biological function
of HSPGs during early zebrafish development is the focus of my laboratory.
Because many steps in zebrafish embryogenesis are similar to those in humans,
the mechanisms and modifiers that I identify may be applicable to better
understanding a wide range of cell-cell signaling events in development and
disease.
Project 1: What is the role of HS core
proteins during gastrulation?
From Drosophila to mouse,
all three cell-cell signaling pathways that regulate early cardiogenesis are in
turn regulated by HSPGs. Consequently, I think the question is not if HSPGs
regulate cardiogenesis, but which specific core proteins are involved and how
do they function? The partial assembly of the zebrafish genome in the last year
has allowed me to clone apparently all 15 of the zebrafish HS cell surface core
proteins. Interestingly, at least 13 are expressed at the beginning of
gastrulation. Of these, only 2 have been described, and they both have distinct
gastrula stage defects in cell migration.
Project 2: Is the HS
fine structure a temporally and spatially permissive sugar code?
Covalently attached
to each core protein is an unbranched chain of 50-100 disaccharide repeats; and
each HS disaccharide can be modified at up to six positions, leading to an
extraordinary level of sequence diversity. Commonly referred to as its fine
structure, the pattern of HS modification over 2-6 disaccharides creates a
specific ligand binding site. In many cases, a cell can only respond to a
cell-cell signaling molecule if it has an appropriate HS fine structure at its
cell surface. Recent results have shown that the HS fine structure changes
during development, suggesting that a cell’s developmental fate is determined
in part by which cell-cell signaling molecules bind to its HS fine structure.
Project 3: Does the core
protein specify its attached HS fine structure?
The current model for
HS synthesis is that the HS fine structure is determined by the complement of
sulfotransferases expressed within the golgi of each cell, regardless of the
core protein to which it might attach. This model is challenged by observations
that modifications in the core protein can alter the interaction of HSPGs with
signaling pathways. However, the HS fine structure might be regulated by the
transition of the core protein through distinct combinations of
sulfotransferase isoforms within the golgi.
<%end if%>
|
|
 |
Laboratories
of the NHLBI |
 |
LDB Home |
 |
|
 |
|
<%
if NIHintranet = "False" and intranet = "Yes" then
'response.write("NIHintranet="+NIHintranet)
' do nothing...
else%>
 |
Sections |
|
|
<%
end if
intranet = "No"
%>
<%
if NIHintranet = "False" and intranet = "Yes" then
'response.write("NIHintranet="+NIHintranet)
' do nothing...
else%>
|
|
<%
end if
intranet = "No"
%>
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
|