Magnetic Resonance
Proton Chemical Exchange Dependent Saturation Transfer (CEST): Evaluation as a Mechanism for Non-Metal Based Exogenous MRI Contrast Agent
K.M. Ward, A.H. Aletras and R.S. Balaban
National Heart Lung and Blood Institute
National Institutes of Health, Bethesda MD, USA.
Click here to download Powerpoint viewer
Click here to view in a Powerpoint slide
Proton "Slow" Exchange |
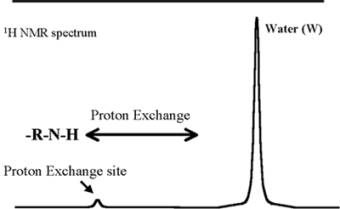 |
Saturation Transfer |
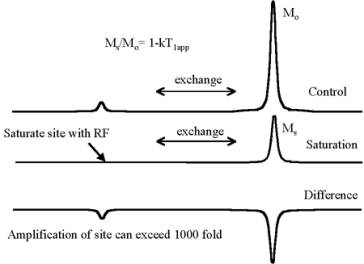 |
Purpose
•To Develop an Exogenous Contrast Agent Based on Chemical Exchange Dependent Saturation Transfer.
•To Evaluate Different Chemical Exchange Sites That Could be Used in the Contrast Agents.
General Experimental Conditions
•Spectroscopy at 300 MHz (Bruker) in 5 mm NMR tubes.
•All experiments conducted at 37oC.
•pH buffers varied, however most comparisons made with 20 mM NaPO4.
•Imaging conducted at 4.0 Tesla at 25 oC (GE whole body system).
Ideal CEST Agent
•1) Remains in slow exchange: tcaDwca>>1.
•2) Minimize tca to enhance saturation transfer: Ms/Mo=[1-(1/tca)T1sat].
•3) This suggests that the larger Dwca the better, since it will support a shorter tca without moving out of the slow exchange domain.
•4) The large Dwca will also improve specificity of the effect (field variations, water T2, MT etc).
•Low toxicity and defined tissue distribution.•
| |
Saturation Transfer Spectrum |
Water Amplitude
(Ms) |
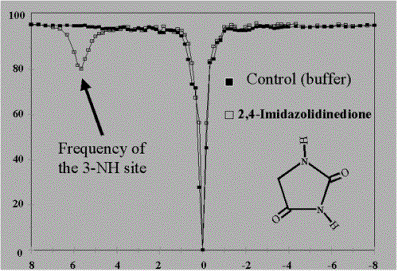 |
| |
Irradiation Offset (ppm) |
Screen of Potential Exchangeable Sites
-OH: Sugars, polymers, Dextran* (small Dw (1.3-1.5ppm))
-NH2 : Amino acids (8) (tca too short at pH 7.0)
-Indole ring –NH: Nucleosides, pyrimidines, purines (good Dw 5-6.5 ppm, adjustable tca)
> 200 compounds evaluated
Barbituric Acid: Effect of Temperature
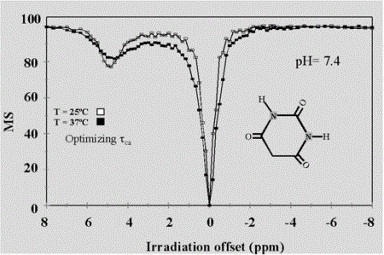
5,6 Dihydrouracil: Effect of pH
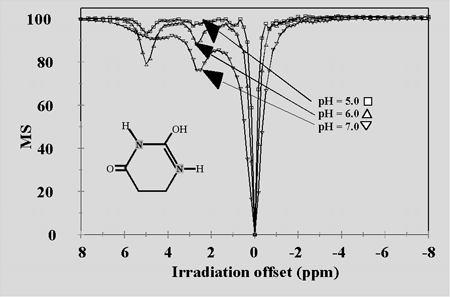
Ratiometric Determination of pH Independent of T1 or [Agent]
![Ratiometric Determination of pH Independent of T1 or [Agent]](images/magnetic-cest6.gif)
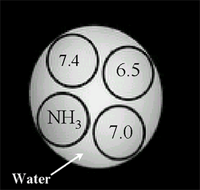
5,6 Dihydrouracil: Effect of [PO4]
![5,6 Dihydrouracil: Effect of [PO4]](images/magnetic-cest7.gif)
Imaging Barbituric Acid Using CEST
 |
 |
Control |
With Irradiation
(Inverse Image) |
62.5 mM |
|
Conclusions
Classes of proton exchange sites were found that could be used for markers of contrast agent concentration, pH or [PO4] using CEST. Several ring –NH exchange sites on were the most useful evaluated with large Dw and variable tca.
Current Limitations
Sensitivity: With the limit of tca and Dw the concentration requirements are on the order of 10 mM to achieve a 10% Ms effect Distribution: Most of the small molecules we have studied to date are quickly cleared to the urine. Toxicity:Relatively unknown. 50 mls of 150 mM Barbiturate has been injected in rabbits with no acute cardiovascular events.
Potential Solutions
Polymerization of exchange sites on single molecule. In practice this has been demonstrated for Dextran: 250 mM Dextrose Ms = 0.9 0.5 mM Dextran (70,000 mW) Ms = 0.85
(i.e. Efficiency is dependent on the number of sites/mole)
Increase proton chemical shift using local dipolar fields (metals). Example is the 70 ppm shift in deoxy-myoglobin. This could significantly extent the tca limit with regards the slow exchange limit. (C. Springer) Other nuclides, Xe.
|