Laboratory of Cell and Tissue Morphodynamics (LCTM)
Clare M Waterman, PhD, Principal Investigator
|
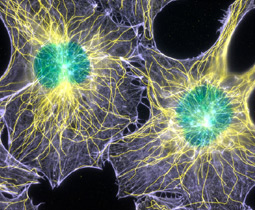
Fluorescence image of filamentous actin and microtubules in mouse fibroblasts cell. 1st place winner of the 2003 Nikon Small World Photomicrography Competition. Image acquired by Torsten Wittmann. View larger image.
|
Welcome to the Laboratory of Cell and Tissue Morphodynamics.
The Laboratory is an intramural research program within the
National Heart Lung and Blood Institute in Bethesda Maryland.
The Chief of the Laboratory is Clare M Waterman, PhD.
Microscopes and Motility: Systems Integration in Directed Cell Migration
The ability of vertebrate cells to directionally move is critical to development, the immune response and wound healing, and its regulation is compromised in, for example, metastatic cancer and vascular disease. Metazoan cells move directionally across an extracellular matrix by a repeating cycle of protrusion of the plasma membrane of the cell edge in the direction of migration, formation of a stable adhesion of the protrusion to the extracellular matrix, pulling against the adhesion sites for translocation of the cell body, and dissolution of older adhesion sites at the trailing edge of the cell to allow rear edge retraction. This necessitates complex and dynamic mechanical interactions between the cell and its extracellular environment that must be coordinated in space and time by physical and biochemical signals. Mechanical cell outputs are mediated in large part by the cytoskeletal polymer systems, actin and microtubules, but also likely involve contributions from other organelle systems in the cell. Our lab uses quantitative microscopy of protein dynamics in living cells and in vitro biochemistry to understand how seemingly distinct cytomechanical systems are integrated with one another to promote the polarized morphogenic activity that drives directed cell movement. We aim to answer questions such as how the microtubule and actin cytoskeletons interact to polarize a motile cell, how the actin cytoskeleton builds specific machines for distinct functions in cell migration, how the dynamics of the endomembrane trafficking systems promote leading edge protrusion, or how the acto-myosin contractile system interfaces with the extracellular matrix via focal adhesions to generate traction force that drives cell movement. To aid our studies of molecular dynamics in cytomechanical systems, we pioneered a method called quantitative Fluorescent Speckle Microscopy (qFSM), which allows quantitative analysis of the dynamics of and interactions between proteins within macromolecular assemblies such as the cytoskeleton and focal adhesions in living cells. Here, I will elaborate on the two main foci of the lab, our work on microtubule/actin interactions in cell migration, and our biologically-driven technology development. Finally, I will discuss how our novel technologies have fueled exciting new biological discoveries that are leading us in new directions in the future.
Microtubule and actin interactions in cell migration
Both of the cytoskeletal polymers, microtubules and filamentous actin (f-actin), are required for directed tissue cell motility. There is extensive evidence that microtubules provide spatial and temporal orchestration of f-actin-based protrusive and contractile activities, however the molecular basis for the interactions between microtubules and f-actin is poorly understood. Our lab has established that microtubules and f-actin exhibit two mechanistic categories of interactions in migrating cells (Rodriguez et al., 2003). "Structural Interactions" are those in which f-actin and microtubules are physically cross-linked, such that microtubules and f-actin directly affect each other's organization and dynamics in cells. "Regulatory Interactions" are those in which the activity of Rho-family small GTPase signaling cascades are spatiotemporally controlled by the assembly and disassembly of microtubules, and at the same time, Rho GTPases regionally co-regulate the dynamics of both microtubules and f-actin to generate asymmetry necessary for migration.
Our lab developed technology (Adams et al., 2003) that allowed us to directly test the hypothesis that microtubules and f-actin exhibit distinct classes of regionally-regulated, specific structural interactions in cytoplasmic extracts (Waterman-Storer et al., 2000), migrating cells (Salmon et al., 2002) and neuronal growth cones (Zhou et al., 2002; Torreano et al., 2005). In collaboration with a Sabbatical researcher in our lab from SUNY Buffalo, Chris Cohan, we showed that in neurons, one specific class of microtubule/f-actin structural interaction is critical to growth cone guidance (Zhou et al., 2002). In epithelial cells, our lab demonstrated that the linking of microtubules to a contractile f-actin network results in asymmetries in microtubule growth dynamics that mirror the asymmetries in f-actin based protrusive and contractile activites that are required for directed cell migration (Gupton et al., 2002).
Our previous studies as a post-doc showed that Rac1 is activated by microtubule growth to promote actin polymerization in lamellipodia, confirming the existence of a specific small GTPase-mediated regulatory interaction from microtubules to f-actin (Waterman-Storer et al., 1999b). Since establishing our research program, we have continued to focus on the Rac1-mediated regulatory interaction between microtubules and f-actin. Our lab first tested the hypothesis that Rac1 could co-regulate microtubule and f-actin dynamics and demonstrated for the first time that Rac1 is a major regulator of microtubule growth (Wittmann et al., 2003). In collaboration with Gary Bokoch (Scripps), we defined Pak kinase and Op18 as its downstream targets (Wittmann et al., 2004). Our lab recently identified a second microtubule-associated downstream target of Rac1, the CLASP proteins, whose association with the lattice of microtubules specifically in the leading edge of migrating cells is regulated by GSK3ß downstream of Rac1 (Wittmann and Waterman-Storer, 2005). The tumor suppressor Adenomatous Polyposis Coli protein (APC) is a third mediator of leading edge microtubule dynamics in migrating cells that we have identified recently in collaboration with Inke Näthke (Dundee University) (Kita et al., 2005). Our lab also has shown that the mechanism by which microtubules promote Rac1 activation involves the directed transport of trans-Golgi-derived secretory vesicles to the leading edge plasma membrane (Prigozhina and Waterman-Storer, 2004).
Quantitative Fluorescent Speckle Microscopy:
A powerful and versatile tool for studying protein dynamics in vivo and in vitro
To facilitate these studies, we developed a new quantitative imaging technology
called Fluorescent Speckle Microscopy (FSM) (Waterman-Storer
et al., 1998). The principle of FSM is simple. FSM is achieved in
living cells by microinjection or expression of a low amount of fluorescently
labeled protein subunits that co-assemble with endogenous unlabeled subunits
into a specific macromolecular structure of interest (Waterman-Storer
and Salmon, 1999; Waterman-Storer
et al., 1998; Waterman-Storer
and Salmon, 1998). Stochastic variations in the number of fluorescent
subunits per resolvable image region results in a "speckled"
appearance of the assembled structure in high-resolution digital fluorescence
light microscope images. In time-lapse FSM, movement and changes in speckle
intensity act as local reporters for movement, assembly and disassembly
of the structure of interest, with time and space resolution in the ten
millisecond and ten nanometer range, respectively. To illustrate the huge
wealth of information contained in FSM images, a single 300 frame FSM
movie of the actin cytoskeleton contains several million flickering and
moving speckles, each of whose intensity and position encodes the local
assembly, disassembly and motion dynamics of the cytoskeleton filaments
in an entire moving cell.
We then established several key collaborations, and over several years
of development, took FSM from a descriptive method to a flexible, robust
and quantitative technology. Since then, FSM has become the premier technology
for sensitively measuring spatiotemporal changes in the composition, assembly,
disassembly or movement of molecular components within cellular machines
from the large ensemble level down to the single molecule level (Danuser
and Waterman-Storer, 2003; Waterman-Storer
and Danuser, 2002). The most challenging task was to derive quantitative
maps of molecular dynamics in cells from FSM image series. Development
of quantitiative FSM (qFSM) was possible by our intense collaboration
with the outstanding computer vision engineer, Gaudenz
Danuser (Scripps) (Ponti
et al., 2003, 2005; Vallotton
et al., 2003, 2004).
We then adapted FSM to spinning disk confocal microscopy and total internal
reflection fluorescence microscopy (Adams
et al., 2003, 2004)
to obtain the optical sectioning advantages of each mode. We were able
to correlate the molecular dynamics within cellular machines to higher
order structure (Gupton
et al., 2005) by collaboration with a structural biologist (Dorit
Hanein, Burnham Inst.). Our lab developed dual-wavelength FSM (Salmon
et al., 2002) to analyze molecular dynamics in pairs of distinct machines.
Recently with the Danuser lab, we have begun to develop correlational
qFSM for analysis of dual-wavelength FSM datasets. This will allow quantitative
mapping in cells of correlations between the dynamics of fluorescent speckles
in two distinct subcellular machines (i.e. focal adhesions and actin (Hu
et al., 2005)) with whole cell morphogenic outputs (i.e. protrusion of
the cell edge). We have also begun to develop fluorescent speckle microrheology
via collaboration with soft condensed matter physicist, Margaret Gardel.
This will allow extraction of viscoelastic moduli of cellular materials
labeled with fluorescent speckles from the thermally induced fluctuations
in speckle position. These accomplishments have allowed FSM to become
a quantitative readout to link molecular and genetic interventions to
changes in molecular dynamics and physical properties of structures to
permit a systematic deciphering of the molecular regulation of dynamic
subcellular machines.
A systems biology approach to integration of subcellular machines
The development and application of this technology has been instrumental
in further stimulating our thinking about how multiple subcellular machines
interact to orchestrate cell migration. We have put this technology to
work to explore the role of specific subellular machines in directed tissue
cell migration. For example, using qFSM in collaboration with the Danuser
lab, we have discovered and characterized two distinct types of cell edge
machines that are built by the actin cytoskeleton in migrating cells,
the protrusive module called the lamellipodium, and the contractile/adhesive
module called the lamellum (Ponti
et al., 2004). We have shown using specific molecular perturbations
that, contrary to the dogma in the field, the lamellum is the machine
that is critical to productive cell movement (Gupton
et al., 2005). We have now begun to dissect the molecular mechanism
of actin filament regulation in the lamellum. Via collaborations with
experts in the biochemistry of different subcellular machines, we have
also recently begun to examine the interactions between the actin cytoskeleton
and the endocytic membrane traffic pathway (with Sandra
Schmid, Scripps; Yarar
et al., 2005), the dynamic molecular interface between the actin cytoskeleton
and focal adhesions (with Mark
Ginsberg, UCSD), the interactions between focal adhesions and cell-cell
adhesions during epithelial-to-mesenchymal transitions (with Martin
Schwartz, Virginia) (de
Rooij et al., 2005) and interactions between microtubules and intermediate
filaments (Bob
Goldman, Northwestern).
Thus, our technology development has facilitated our discovery and mechanistic
characterization of structural and regulatory cross-talk between several
pairs of subcellular machines that are critical to orchestrating the morphogenic
cell changes mediating directed cell migration. This has illuminated the
clear realization that only through development of new technologies to
analyze the interactions between multiple subcellular machines and physical
outputs will a thorough understanding of the complex orchestration of
cell morphogenic events in cell migration be achieved. Over the next five
years, we plan to continue to focus on the molecular mechanisms mediating
the interactions between microtubules and actin and to determine the requirement
for these interactions in directed cell migration. We will additionally
focus on analyzing the dynamic interface between the focal adhesions and
the actin cytoskeleton. Here, we plan to use correlational qFSM, in combination
with traction force microscopy and optical trapping force spectroscopy
to probe the molecular mechanism of force production in cell migration.
With the Danuser lab, we will continue to push the development of FSM
technology, including adapting it for high throughput, high content screening,
and combining FSM with spectral imaging to allow the correlation of multiple
cytomechanical systems with cell morphogenic and physical outputs.