|
Amyloidogenic Proteins
α-Synuclein
Study of the amyloidogenic protein α-synuclein (α-syn) is of particular interest in our laboratory. Understanding the effects of local environment on the conformation of α-syn is of great importance because its aggregation in vivo results in amyloid deposits in the brain called Lewy Bodies that are a hallmark of Parkinsonís disease (PD). While it is clear that α-syn plays a central role in the etiology of PD, its role as a neurotoxic agent remains elusive.
One aim of our lab is to elucidate how α-syn conformation and aggregation behavior change upon membrane interaction. Armed with several spectroscopic probes including far UV circular dichroism and steady-state and time-resolved fluorescence techniques we study the response of α-syn to both synthetic phospholipid vesicles and isolated synaptic membranes. Through these studies we seek insights into the role of synaptic organelles on α-syn conformational change.
In addition, studies on the role of external environmental factors on α-syn conformation and in vivo experiments are being conducted. Particularly, effects of redox active metals, such as copper(II/I), on α-syn are of interest because metals are known to promote aggregation in vitro. Cellular studies are aimed at uncovering the intracellular localization and structure of modified α-syn in live, dopaminergic, neuroblastoma cells using fluorescence microscopy.

Schematic of environmentally dependent secondary structural changes for α-synuclein. Unstructured in buffer (left), α-helical in the presence of membranes (middle), and β-sheet in amyloid fibrils (right).
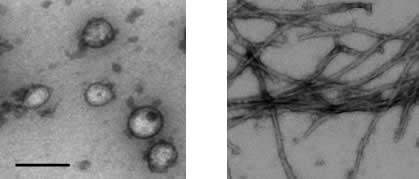
Electron micrograph images of isolated mouse synaptic vesicles (left) fibrils of aggregated α-synuclein (right). Scale bar 100 nm
Confocal microscopy image of fixed M17 neuroblastoma cells. Green = G7C-fluorescein labeled α-synuclein. Blue = nuclear DNA stained with DAPI. Bar = 10 microns.
|