|
Ion Receptors and Transporters
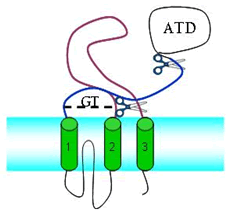 |
| A single iGluR subunit has 3 domains, amino terminal (ATD), ligand binding (purple and blue)and 3 transmembrane helices (green). The amino terminal and transmembrane domains can be removed and the soluble ligand binding domain investigated independently. |
Ionotropic Glutamate Receptors
Located in the postsynaptic density of the central nervous system, ionotropic glutamate receptors (iGluRs) are responsible for transmission of excitatory neurological signals across the synapse. Chemical neurotransmitters released from the presynaptic terminal bind to the extracellular domain of iGluRs. Binding results in a conformational change in the extracellular domain. This conformational change causes opening of the transmembrane channel to allow ions to flow across the membrane.
The modularity of this protein allows for studies on isolated extracellular subunits. Currently, we are developing optical spectroscopic probes to investigate the dynamics of ligand binding to iGluRs. Our ultimate goal is to extend these experiments to investigate the coupling of ligand binding to channel opening (protein allostery) by performing laser spectroscopic measurements on a intact receptor.
Glutamate Transporters
Following neurotransmitter release from the presynaptic terminal it is necessary to clear molecules from the synapse to avoid the neurotoxic effects of overexcitation in the postsynaptic neuron. This is accomplished by glutamate transporters located in the glial cells adjacent to the synapse. These transmembrane proteins couple the uptake of a glutamate anion with the transport of three sodium ions and a proton out of the cell which is followed by the reverse transport of a potassium ion.
 |
| Side view (left) and top view (right) of a bacterial transporter from Pyrococcus horikoshii, a homotrimer spanning the cell membrane of glial cells. (1xfh.pdb) |
We seek to take advantage of a structurally characterized bacterial homolog of these crucial transporters which are easily reconstitutable and amendable to a vast array of biochemical and spectroscopic studies. Specifically, we are probing the steps involved in the gating mechanism of the central pore. (In collaboration with Joseph Mindell, NINDS)
|